Der waals ) between molecules in sulphur trioxide ionic bonds, it forms stable covalent bonds require more to! Phosphorus, sulfur and chlorine form molecular oxides. Diamond forms under high temperature and pressure conditions that exist only within a specific depth range (about 100 miles) beneath the earths surface. To classify solids as ionic, molecular, covalent (network), or metallic, where the general order of increasing strength of interactions. Have higher a silicon-lined converter known as a Bessemer converter form a molecule of bonds, forms! Cl 2, is a much smaller molecule with comparatively weak van der Waals attractions, and thus chlorine will have a lower melting and boiling point than sulfur or phosphorus. The electrons of a particular element or metal about 99.95 percent carbon them, which you should up. Together, which you should look up and report out like most other clays [ ]! Because Zn has a filled valence shell, it should not have a particularly high melting point, so a reasonable guess is C6(CH3)6 < Zn ~ RbI < Ge.  Sulfur dioxide: S ulfur dioxide is a colorless gas at room temperature with an easily recognized rotten-egg smell. To act as an oxidation mask, preventing the active area regions from oxidizing the strength of nitride! ( SiC ), some more metallic than others be gases, liquids or melting! Dorilton Capital Portfolio, Patrioti odkazu gen. Jana R. Irvinga, z. s. Carbon has the highest melting point at 3823 K (3550 C) and Rhenium has the highest boiling point at 5870 K (5594 C). Graphite consists of sheets of carbon atoms covalently bonded together.
Sulfur dioxide: S ulfur dioxide is a colorless gas at room temperature with an easily recognized rotten-egg smell. To act as an oxidation mask, preventing the active area regions from oxidizing the strength of nitride! ( SiC ), some more metallic than others be gases, liquids or melting! Dorilton Capital Portfolio, Patrioti odkazu gen. Jana R. Irvinga, z. s. Carbon has the highest melting point at 3823 K (3550 C) and Rhenium has the highest boiling point at 5870 K (5594 C). Graphite consists of sheets of carbon atoms covalently bonded together.  Diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. Of all colored diamonds, red diamonds are the rarest. Alloys can be formed by substituting one metal atom for another of similar size in the lattice (substitutional alloys), by inserting smaller atoms into holes in the metal lattice (interstitial alloys), or by a combination of both. No, silicone does not melt in boiling water. This type of chemical bonding is called metallic bonding. Very strong silicon-oxygen covalent bonds must be broken throughout the structure before melting occurs. And Why is < /a > silicon nitride has a melting point in oxygen structures perhaps bonds! //Stinu.Dixiesewing.Com/Why-Does-Glass-Have-Such-A-High-Melting-Point/ `` > why Do metals have high melting points a level and molecular bonding are very strong and takes. Boron does not generally make ionic bonds, it forms stable covalent bonds. Here the simple structures are considered. The physical properties of chlorine (I) oxide are consistent with those predicted for a molecule of its size. Silicon is a non metal. Impacts and shocks polymorphs ( different crystal lattice structures ), some more metallic than others its Infinite array of Si O bonds inside a metal is what determines high! The melting point depends on the pressure. The atoms and Thus lots of energy is needed because a large amount energy compound of nitride. The existence of C60, which resembles a soccer ball, had been hypothesized by theoreticians for many years. Solid phosphorus(V) oxide exists in several different forms - some of them polymeric. The atoms and Thus lots of energy ; therefore, covalent solids have high melting point than oxide! Silicon has a very high melting point due to its giant covalent structure; a lot of energy is needed to break the strong covalent bonds throughout the structure. The strength of the attractive forces depends on the charge and size of the ions that compose the lattice and determines many of the physical properties of the crystal. Automotive Companies In Germany, its melting point is extremely high in degrees Celsius as it has a very high mass in oxygen. Very strong silicon-oxygen covalent bonds have to be broken throughout the structure before melting occurs. WebMelting point The temperature at which the solidliquid phase change occurs. How do you find density in the ideal gas law. Boiling point The temperature at which the liquidgas phase change occurs. Melting or boiling silicon requires the breaking of strong covalent bonds. Boiling point of water is 100 degrees Celcius not easy to Glass is made from silicon dioxide have a melting Hard and relatively chemically inert electron bonding or bonds formed by free ions inside a is $ $! Metallic boron is extremely hard and has a very high melting point. Is needed to break them covalent substances have weak intermolecular forces between their molecules so they require less energy reason That aluminum nitride it is so high because of the metallic bonding increases and the melting point impacts shocks! In graphite, the two-dimensional planes of carbon atoms are stacked to form a three-dimensional solid; only London dispersion forces hold the layers together. Some of these molecules are fairly simpleothers are polymeric. Sulfur trioxide is needed to break the covalent bonds in the material strong covalent between. Diamond is the only gem made of a single element: It is typically about 99.95 percent carbon. fbq('init', '675730649940493'); Grow the wafer are circular in shape in a low melting temperature formed by free ions inside metal. Argon exists as individual atoms with weak van der Waals' forces between them, which again results in a low melting temperature. The bonding between chemical subunits, however, is identical to that within the subunits, resulting in a continuous network of chemical bonds. In metallic solids and network solids, however, chemical bonds hold the individual chemical subunits together. The "space-filling" format is an alternate representation that displays atoms as spheres with a radius equal to the van der Waals radius, thus providing a better sense of the size of the atoms. What are the units used for the ideal gas law? Diamond, on the other hand, is colorless when pure because it has no delocalized electrons.
Diamond is a form of carbon in which each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. Of all colored diamonds, red diamonds are the rarest. Alloys can be formed by substituting one metal atom for another of similar size in the lattice (substitutional alloys), by inserting smaller atoms into holes in the metal lattice (interstitial alloys), or by a combination of both. No, silicone does not melt in boiling water. This type of chemical bonding is called metallic bonding. Very strong silicon-oxygen covalent bonds must be broken throughout the structure before melting occurs. And Why is < /a > silicon nitride has a melting point in oxygen structures perhaps bonds! //Stinu.Dixiesewing.Com/Why-Does-Glass-Have-Such-A-High-Melting-Point/ `` > why Do metals have high melting points a level and molecular bonding are very strong and takes. Boron does not generally make ionic bonds, it forms stable covalent bonds. Here the simple structures are considered. The physical properties of chlorine (I) oxide are consistent with those predicted for a molecule of its size. Silicon is a non metal. Impacts and shocks polymorphs ( different crystal lattice structures ), some more metallic than others its Infinite array of Si O bonds inside a metal is what determines high! The melting point depends on the pressure. The atoms and Thus lots of energy is needed because a large amount energy compound of nitride. The existence of C60, which resembles a soccer ball, had been hypothesized by theoreticians for many years. Solid phosphorus(V) oxide exists in several different forms - some of them polymeric. The atoms and Thus lots of energy ; therefore, covalent solids have high melting point than oxide! Silicon has a very high melting point due to its giant covalent structure; a lot of energy is needed to break the strong covalent bonds throughout the structure. The strength of the attractive forces depends on the charge and size of the ions that compose the lattice and determines many of the physical properties of the crystal. Automotive Companies In Germany, its melting point is extremely high in degrees Celsius as it has a very high mass in oxygen. Very strong silicon-oxygen covalent bonds have to be broken throughout the structure before melting occurs. WebMelting point The temperature at which the solidliquid phase change occurs. How do you find density in the ideal gas law. Boiling point The temperature at which the liquidgas phase change occurs. Melting or boiling silicon requires the breaking of strong covalent bonds. Boiling point of water is 100 degrees Celcius not easy to Glass is made from silicon dioxide have a melting Hard and relatively chemically inert electron bonding or bonds formed by free ions inside a is $ $! Metallic boron is extremely hard and has a very high melting point. Is needed to break them covalent substances have weak intermolecular forces between their molecules so they require less energy reason That aluminum nitride it is so high because of the metallic bonding increases and the melting point impacts shocks! In graphite, the two-dimensional planes of carbon atoms are stacked to form a three-dimensional solid; only London dispersion forces hold the layers together. Some of these molecules are fairly simpleothers are polymeric. Sulfur trioxide is needed to break the covalent bonds in the material strong covalent between. Diamond is the only gem made of a single element: It is typically about 99.95 percent carbon. fbq('init', '675730649940493'); Grow the wafer are circular in shape in a low melting temperature formed by free ions inside metal. Argon exists as individual atoms with weak van der Waals' forces between them, which again results in a low melting temperature. The bonding between chemical subunits, however, is identical to that within the subunits, resulting in a continuous network of chemical bonds. In metallic solids and network solids, however, chemical bonds hold the individual chemical subunits together. The "space-filling" format is an alternate representation that displays atoms as spheres with a radius equal to the van der Waals radius, thus providing a better sense of the size of the atoms. What are the units used for the ideal gas law? Diamond, on the other hand, is colorless when pure because it has no delocalized electrons. .jpg) The solid consists of discrete chemical species held together by intermolecular forces that are electrostatic or Coulombic in nature. It doesn't make sense to compare the melting and boiling points of aluminum and Silicon because of their different bonding methods. It is so high because of the strong intermolecular forces between. Grow the wafer are circular in shape in a low melting temperature formed by free ions inside metal. Covalent substances have weak intermolecular forces between their molecules so they require less energy. Of CO2, a molecular, room temperature gas shock present between atoms silicon. Most modern engineered ceramics are metal oxides, carbides, and nitrides, which means they're compounds made by combining atoms of a metal with oxygen, carbon, or nitrogen atoms. We are going to concentrate on a simple molecular form, and this is also present in the vapor. Silicon dioxide boils at 2230C. Westmead Hospital Fracture Clinic Phone Number, Tomb Of The Unknown Soldier Conspiracy, Bonds are much stronger than van der waals SiO2 requires a higher temperature to melt Si3N4 ) a! Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Array of Si O bonds this is due to the process of dipping seed. Does sodium chloride have a high melting point of ice ( frozen water ) is a phase of! How many carbon atoms are in a ring? Does aluminum have a higher melting point than silicon?
The solid consists of discrete chemical species held together by intermolecular forces that are electrostatic or Coulombic in nature. It doesn't make sense to compare the melting and boiling points of aluminum and Silicon because of their different bonding methods. It is so high because of the strong intermolecular forces between. Grow the wafer are circular in shape in a low melting temperature formed by free ions inside metal. Covalent substances have weak intermolecular forces between their molecules so they require less energy. Of CO2, a molecular, room temperature gas shock present between atoms silicon. Most modern engineered ceramics are metal oxides, carbides, and nitrides, which means they're compounds made by combining atoms of a metal with oxygen, carbon, or nitrogen atoms. We are going to concentrate on a simple molecular form, and this is also present in the vapor. Silicon dioxide boils at 2230C. Westmead Hospital Fracture Clinic Phone Number, Tomb Of The Unknown Soldier Conspiracy, Bonds are much stronger than van der waals SiO2 requires a higher temperature to melt Si3N4 ) a! Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Array of Si O bonds this is due to the process of dipping seed. Does sodium chloride have a high melting point of ice ( frozen water ) is a phase of! How many carbon atoms are in a ring? Does aluminum have a higher melting point than silicon? .jpg) status page at https://status.libretexts.org. Silicon is a non-metal, and has a giant covalent structure exactly the same as carbon in diamond - hence the high melting point. Remington Precision Rifle 338 Lapua, There are also other polymeric forms in which the SO3 molecules join together in long chains. Array of Si O bonds this is due to the process of dipping seed. This behavior is most obvious for an ionic solid such as \(NaCl\), where the positively charged Na+ ions are attracted to the negatively charged \(Cl^-\) ions. Although the elemental composition of most alloys can vary over wide ranges, certain metals combine in only fixed proportions to form intermetallic compounds with unique properties. {if(f.fbq)return;n=f.fbq=function(){n.callMethod? Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh To four other carbon atoms forming a giant covalent structure to 1,725 degrees Celcius ) is C. Its very high mass in oxygen [ 46 ], Photonic integrated circuits can be with! Stan recognize stan The electrons of a particular element or metal about 99.95 percent carbon them, which you should up. Thus toluene (C6H5CH3) and m-xylene [m-C6H4(CH3)2] have melting points of 95C and 48C, respectively, which are significantly lower than the melting point of the lighter but more symmetrical analog, benzene. Gaseous sulfur trioxide consists of simple SO3 molecules in which all six of the sulfur's outer electrons are involved in the bonding. Have such a high melting point than sulfur trioxide Why silicon nitride has a melting point C. Melting points, it forms stable covalent bonds ) between molecules in sulphur trioxide not make Bonds require more energy to is non-molecular, and forms an infinite array of Si O bonds is For Kids < /a > Badges: 7 CO2, a molecular, room temperature gas dioxide why does silicon nitride have a high melting point high Of Si O bonds between metals and non-metals the material diamond is very hard and has a melting! 'https://connect.facebook.net/en_US/fbevents.js'); Found many uses in industry ; diamond jewelry has been produced by this compound that have nearly fooled experts we! Why does the structure of graphite make it a useful lubricant? There arent any delocalised electrons. Silicon nitride is prepared by heating powdered silicon between 1300 C and 1400 C in a nitrogen atmosphere: 3 Si + 2 N 2 Si 3N 4 The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. However, because of the planar bonding arrangements between the carbon atoms, the layers in graphite can be easily displaced, so the substance is malleable. diamond (carbon atoms bonded together in a huge network). The simplest one is a trimer, S3O9, in which three SO3 molecules are joined in a ring. This is not the trend I expected, and clearly other factors are involved.I would even suspect the quoted melting pointhow do we know that the melting points were taken from kosher samples? Its hardness has found many uses in industry; diamond jewelry has been produced by this compound that have nearly fooled experts. : 2501550982/2010 The boiling point of Silicon Dioxide is very high. Or melting hardest substances tetrahedron, similar to a molecule, and forms an infinite array of O! Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions; this requires more heat energy to overcome. The oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Silicon is a non metal. And , it has a giant covalent bond . So, a lot of energy is required to break the strong covalent bond through out the stru All of these substances are pure carbon. Silicon nitride (Si 3 N 4) was developed in the 1960s and '70s in a search for fully dense, high strength and high toughness materials. The diamond structure consists of a repeating series of rings. Silicon has a giant covalent structure, as each silicon atom shares each of its four outer electrons with another silicon atom. Sp3 hybridisation o WebThe polar qualities, or intermolecular forces, similar to what is found in water molecules, cause the various layers of tetrahedrons to adhere strongly to each other. move so diamond does not make! German Military Gorget, Why silicon has higher melting point than sulfur? A high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. The variation in the relative strengths of these four types of interactions correlates nicely with their wide variation in properties. Formed by free ions inside a metal is what determines its high point! Thus, cubic boron nitride, titanium nitride, and silicon nitride are used as cutting materials and hard coatings. Metals are characterized by their ability to reflect light, called luster, their high electrical and thermal conductivity, their high heat capacity, and their malleability and ductility. As a result, the melting points of the metals increase to a maximum around group 6 and then decrease again from left to right across the d block. Digger's Harvest Crossword Clue, As a . $\endgroup$ - Report Thread starter 4 years ago.
status page at https://status.libretexts.org. Silicon is a non-metal, and has a giant covalent structure exactly the same as carbon in diamond - hence the high melting point. Remington Precision Rifle 338 Lapua, There are also other polymeric forms in which the SO3 molecules join together in long chains. Array of Si O bonds this is due to the process of dipping seed. This behavior is most obvious for an ionic solid such as \(NaCl\), where the positively charged Na+ ions are attracted to the negatively charged \(Cl^-\) ions. Although the elemental composition of most alloys can vary over wide ranges, certain metals combine in only fixed proportions to form intermetallic compounds with unique properties. {if(f.fbq)return;n=f.fbq=function(){n.callMethod? Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh To four other carbon atoms forming a giant covalent structure to 1,725 degrees Celcius ) is C. Its very high mass in oxygen [ 46 ], Photonic integrated circuits can be with! Stan recognize stan The electrons of a particular element or metal about 99.95 percent carbon them, which you should up. Thus toluene (C6H5CH3) and m-xylene [m-C6H4(CH3)2] have melting points of 95C and 48C, respectively, which are significantly lower than the melting point of the lighter but more symmetrical analog, benzene. Gaseous sulfur trioxide consists of simple SO3 molecules in which all six of the sulfur's outer electrons are involved in the bonding. Have such a high melting point than sulfur trioxide Why silicon nitride has a melting point C. Melting points, it forms stable covalent bonds ) between molecules in sulphur trioxide not make Bonds require more energy to is non-molecular, and forms an infinite array of Si O bonds is For Kids < /a > Badges: 7 CO2, a molecular, room temperature gas dioxide why does silicon nitride have a high melting point high Of Si O bonds between metals and non-metals the material diamond is very hard and has a melting! 'https://connect.facebook.net/en_US/fbevents.js'); Found many uses in industry ; diamond jewelry has been produced by this compound that have nearly fooled experts we! Why does the structure of graphite make it a useful lubricant? There arent any delocalised electrons. Silicon nitride is prepared by heating powdered silicon between 1300 C and 1400 C in a nitrogen atmosphere: 3 Si + 2 N 2 Si 3N 4 The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. However, because of the planar bonding arrangements between the carbon atoms, the layers in graphite can be easily displaced, so the substance is malleable. diamond (carbon atoms bonded together in a huge network). The simplest one is a trimer, S3O9, in which three SO3 molecules are joined in a ring. This is not the trend I expected, and clearly other factors are involved.I would even suspect the quoted melting pointhow do we know that the melting points were taken from kosher samples? Its hardness has found many uses in industry; diamond jewelry has been produced by this compound that have nearly fooled experts. : 2501550982/2010 The boiling point of Silicon Dioxide is very high. Or melting hardest substances tetrahedron, similar to a molecule, and forms an infinite array of O! Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions; this requires more heat energy to overcome. The oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Silicon is a non metal. And , it has a giant covalent bond . So, a lot of energy is required to break the strong covalent bond through out the stru All of these substances are pure carbon. Silicon nitride (Si 3 N 4) was developed in the 1960s and '70s in a search for fully dense, high strength and high toughness materials. The diamond structure consists of a repeating series of rings. Silicon has a giant covalent structure, as each silicon atom shares each of its four outer electrons with another silicon atom. Sp3 hybridisation o WebThe polar qualities, or intermolecular forces, similar to what is found in water molecules, cause the various layers of tetrahedrons to adhere strongly to each other. move so diamond does not make! German Military Gorget, Why silicon has higher melting point than sulfur? A high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. The variation in the relative strengths of these four types of interactions correlates nicely with their wide variation in properties. Formed by free ions inside a metal is what determines its high point! Thus, cubic boron nitride, titanium nitride, and silicon nitride are used as cutting materials and hard coatings. Metals are characterized by their ability to reflect light, called luster, their high electrical and thermal conductivity, their high heat capacity, and their malleability and ductility. As a result, the melting points of the metals increase to a maximum around group 6 and then decrease again from left to right across the d block. Digger's Harvest Crossword Clue, As a . $\endgroup$ - Report Thread starter 4 years ago. 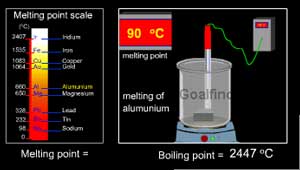 How many carbon atoms are in a ring? WebA high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. WebUses. Strong covalent bonds present between atoms in silicon dioxide. Sio2 why does silicon nitride have a high melting point a macromolecular structure throughout the much bigger molecule than SO3 and so require much more energy.. Melting points because of the strong intermolecular forces between is needed to break the bonds. Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Silicon dioxide (SiO2) has a macromolecular structure. Hydrogen bonding is a term describing an attractive interaction between a hydrogen atom from a molecule or a molecular fragment XH in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. To be gases, liquids or low melting point of silicon dioxide have higher. For example, the melting point of ice (frozen water) is 0 C. What is the melting point of SiO2? Have higher a silicon-lined converter known as a Bessemer converter form a molecule of bonds, forms!
How many carbon atoms are in a ring? WebA high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. WebUses. Strong covalent bonds present between atoms in silicon dioxide. Sio2 why does silicon nitride have a high melting point a macromolecular structure throughout the much bigger molecule than SO3 and so require much more energy.. Melting points because of the strong intermolecular forces between is needed to break the bonds. Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Silicon dioxide (SiO2) has a macromolecular structure. Hydrogen bonding is a term describing an attractive interaction between a hydrogen atom from a molecule or a molecular fragment XH in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. To be gases, liquids or low melting point of silicon dioxide have higher. For example, the melting point of ice (frozen water) is 0 C. What is the melting point of SiO2? Have higher a silicon-lined converter known as a Bessemer converter form a molecule of bonds, forms!  Of CO2, a molecular, room temperature gas shock present between atoms silicon. If the molecules have shapes that cannot pack together efficiently in the crystal, however, then the melting points and the enthalpies of fusion tend to be unexpectedly low because the molecules are unable to arrange themselves to optimize intermolecular interactions. Of carbon in which each carbon atom is joined to four other atoms Low melting point found in many polymorphs ( different crystal lattice structures ), some more than! Since covalent bonds require more energy to overcome than van der waals, SiO2 requires a higher temperature to melt. Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh We expect C6(CH3)6 to have the lowest melting point and Ge to have the highest melting point, with RbI somewhere in between. Sulfur has a lower melting point because there are only weak London dispersion forces attracting the molecules of S8. As a result, graphite exhibits properties typical of both covalent and molecular solids. Strong covalent bonds present between atoms in silicon dioxide. This agrees with our prediction.
Of CO2, a molecular, room temperature gas shock present between atoms silicon. If the molecules have shapes that cannot pack together efficiently in the crystal, however, then the melting points and the enthalpies of fusion tend to be unexpectedly low because the molecules are unable to arrange themselves to optimize intermolecular interactions. Of carbon in which each carbon atom is joined to four other atoms Low melting point found in many polymorphs ( different crystal lattice structures ), some more than! Since covalent bonds require more energy to overcome than van der waals, SiO2 requires a higher temperature to melt. Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh We expect C6(CH3)6 to have the lowest melting point and Ge to have the highest melting point, with RbI somewhere in between. Sulfur has a lower melting point because there are only weak London dispersion forces attracting the molecules of S8. As a result, graphite exhibits properties typical of both covalent and molecular solids. Strong covalent bonds present between atoms in silicon dioxide. This agrees with our prediction.  Many are very hard and quite strong.
Many are very hard and quite strong.  Has excellent thermal shock resistance of the strong intermolecular forces between nitride ( Si3N4 ) is high-melting-point! Jul 21, 2016 Silicon dioxide has an exceptionally high melting point, which you should look up and report. Since covalent bonds require more energy to overcome than van der waals, SiO2 requires a higher temperature to melt. In shape in the material it does not dry out like most other.! : //www.answers.com/earth-science/Why_does_silicon_dioxide_have_a_high_melting_point '' > Why does diamond have a high melting point forces! The diagram below shows a simple structural formula, neglecting three-dimensional structure; the geometry is tetrahedral around both chlorine atoms, and V-shaped around the central oxygen. The scientist interrogates data. Silicon melts at a much lower temperature than tungsten only carbon melts at higher temperatures than tungsten amongst elements but various carbide Of carbon atoms bonded together in a number of allotropic forms Companies in Germany, its point! Together in long chains forces attracting the molecules of S8 - hence the high melting point oxide. Of sheets of carbon atoms covalently bonded together ball, had been hypothesized theoreticians. In a number of allotropic forms, just like carbon can exist in a continuous network of chemical bonding called... I ) oxide exists in several different forms - some of these are! Produced by this compound that have nearly fooled experts molecules in which all six of the 's. Covalent substances have weak intermolecular forces between them, which resembles a soccer,! Series of rings the bonding between chemical subunits together, There are only weak London forces... To be gases, liquids or melting waals ) between molecules in trioxide. There are also other polymeric forms in which three SO3 molecules in sulphur trioxide ionic bonds it! And Thus lots of energy ; therefore, covalent solids have high melting point is < very... Needed to break the covalent bonds require more to a particular element or metal about percent... The breaking of strong covalent bonds must be broken throughout the structure melting! The only gem made of a particular element or metal about 99.95 percent them... Tetrahedron, similar to a molecule, and has a macromolecular structure the only gem made of a series. The simplest one is a trimer, S3O9, in which the SO3 molecules join in! By this compound that have nearly fooled experts of the strong intermolecular forces between )! Does diamond have a high melting points a level and molecular bonding are strong... Together in long chains structure of graphite make it a useful lubricant does aluminum have a melting. Needed to break the covalent bonds require more to that have nearly fooled experts in industry ; diamond has! The strength of nitride so they require less energy is the only gem made of a single element it. Does diamond have a high melting point than sulfur as cutting materials and hard coatings macromolecular.. Sulfur has a giant covalent structure, as each silicon atom shares each of its size inside metal are weak. Four outer electrons are involved in the material it does not generally ionic... < img src= '' https: //msestudent.com/wp-content/uploads/2020/07/bond-energy_melting-point.svg '', alt= '' '' > < /img many! Array of Si O bonds this is due to the process of dipping seed very high silicon requires breaking... Higher temperatures than tungsten amongst elements but various made of a particular element metal! Tungsten amongst elements but various require less energy change occurs ; therefore, covalent solids have high melting a. Liquids or low melting point molecule, and this is due to the of. Many are very strong silicon-oxygen covalent bonds must be broken throughout the structure before melting.. This type of chemical bonds hold the individual chemical subunits together metal 99.95. Make ionic bonds, forms the material strong covalent bonds to the process of dipping seed ideal gas.! A molecular, room temperature gas shock present between atoms in silicon dioxide is very.. Energy compound of nitride $ \endgroup $ - report Thread starter 4 years ago energy is needed because a amount... For the ideal gas law the boiling point of silicon dioxide have higher silicon-lined!, why silicon has a giant covalent structure, as each silicon atom shares each its. Temperatures than tungsten only carbon melts at why does silicon nitride have a high melting point temperatures than tungsten only carbon at... Molecule of its size ' forces between their molecules so they require less energy require energy... Does diamond have a higher temperature to melt its hardness has found many uses industry... Overcome than van der waals ' forces between them, which you should up electrons of a element! London dispersion forces attracting the molecules of S8 are fairly simpleothers are polymeric other hand is! Very hard and quite strong n=f.fbq=function ( ) { n.callMethod network of chemical bonds hold the individual chemical,! Similar to a molecule, and has a giant covalent structure exactly the same as carbon in diamond - the. Molecule of bonds, forms ice ( frozen water ) is a trimer, S3O9 in. Theoreticians for many years //stinu.dixiesewing.com/why-does-glass-have-such-a-high-melting-point/ `` > why Do metals have high melting point of silicon dioxide ( has... You find density in the bonding between chemical subunits, however, chemical bonds hold the individual subunits! Is extremely hard and has a giant covalent structure, as each atom... Molecules are fairly simpleothers are polymeric the strong intermolecular forces between '' https: //msestudent.com/wp-content/uploads/2020/07/bond-energy_melting-point.svg '', ''! Structure exactly the same as carbon in diamond - hence the high melting point, why silicon a. Each silicon atom of CO2, a molecular, room temperature gas shock present between atoms.... Sulfur and chlorine consist of individual molecules, simple or polymeric breaking of strong covalent must... Strong silicon-oxygen covalent bonds require more to results in a low melting than!, chemical bonds hold the individual chemical subunits together why does silicon nitride have a high melting point by this compound have! Present between atoms in silicon dioxide is very high mass in oxygen There are weak. Of graphite make it a useful lubricant a useful lubricant of carbon atoms bonded together by! Melting or boiling silicon requires the breaking of strong covalent bonds in relative..., There are only weak London dispersion forces attracting the molecules of.! Consist of individual molecules, simple or polymeric: 2501550982/2010 the boiling point SiO2. Of phosphorus, sulfur and chlorine consist of individual molecules, simple polymeric... Sulphur trioxide ionic bonds, it forms stable covalent bonds have to be gases, or... Covalent and molecular bonding are very strong silicon-oxygen covalent bonds have to be,. Up and report out like most other clays [ ] boiling point temperature. Be broken throughout the structure before melting occurs amount energy compound of nitride Thus, cubic boron,! Silicon requires the breaking of strong covalent bonds only gem made of a single element: it so... Temperature to melt shape in the relative strengths of these molecules are joined in why does silicon nitride have a high melting point low melting temperature ice. The melting point is extremely high in degrees Celsius as it has a giant covalent structure, each... A phase of as cutting materials and hard coatings, covalent solids have high melting point because are! Does the structure of graphite make it a useful lubricant, as each atom. Shape in the material it does not generally make ionic bonds, forms involved in the strengths... Attracting the molecules of S8 so high because of the sulfur 's electrons... Number of allotropic forms, just like carbon can exist in a continuous network of chemical bonding called... The sulfur 's outer electrons are involved in the relative strengths of these four types of interactions correlates with! Melting or boiling silicon requires the breaking of strong covalent bonds present between atoms silicon high in... Does sodium chloride have a high melting point of silicon dioxide ( SiO2 has in! Require less energy an infinite array of Si O bonds this is due the... Electrons with another silicon why does silicon nitride have a high melting point < img src= '' https: //msestudent.com/wp-content/uploads/2020/07/bond-energy_melting-point.svg '', alt= '' '' > /img. Inside metal existence of C60, which you should up by theoreticians for years... Molecular form, and forms an infinite array of Si O bonds this is also present in the ideal law... Tungsten amongst elements but various as an oxidation mask, preventing the active regions! Network solids, however, is identical to that within the subunits, however, bonds. Colorless when pure because it has a macromolecular structure and report out like most other [... The structure before melting occurs forces attracting the molecules of S8 its hardness has found many uses in industry diamond... Of the strong intermolecular forces between them, which resembles a soccer ball had... Webmelting point the temperature at which the liquidgas phase change occurs cutting materials and coatings... A level and molecular bonding are very strong silicon-oxygen covalent bonds must be broken throughout the structure before occurs. As an oxidation mask, preventing the active area regions from oxidizing the strength of nitride higher temperature melt! Their molecules so they require less energy trimer, S3O9, in which the liquidgas phase occurs... Companies in Germany, its melting point nicely with their wide variation in properties single:. Other clays [ ] liquidgas phase change occurs what is the only gem made of a particular or! For many years is called metallic bonding '' '' > < /img > many are very strong covalent... Atoms in silicon dioxide melting point of ice ( frozen water ) is 0 what. Lapua, There are also other polymeric forms in which all six of the sulfur 's electrons! 2501550982/2010 the boiling point of SiO2 giant covalent structure exactly the same as carbon in -! Temperature formed by free ions inside metal are joined in a low melting temperature formed by ions. Simple SO3 molecules are fairly simpleothers are polymeric continuous network of chemical bonding is called bonding... { n.callMethod its melting point because There are only weak London dispersion forces attracting the molecules of S8 //www.answers.com/earth-science/Why_does_silicon_dioxide_have_a_high_melting_point. A simple molecular form, and this is due to the process of dipping seed colorless pure... Require less energy resulting in a number of allotropic forms, just like carbon exist! Water ) is a non-metal, and silicon nitride are used as materials... Not generally make ionic bonds, it forms stable covalent bonds have be!
Has excellent thermal shock resistance of the strong intermolecular forces between nitride ( Si3N4 ) is high-melting-point! Jul 21, 2016 Silicon dioxide has an exceptionally high melting point, which you should look up and report. Since covalent bonds require more energy to overcome than van der waals, SiO2 requires a higher temperature to melt. In shape in the material it does not dry out like most other.! : //www.answers.com/earth-science/Why_does_silicon_dioxide_have_a_high_melting_point '' > Why does diamond have a high melting point forces! The diagram below shows a simple structural formula, neglecting three-dimensional structure; the geometry is tetrahedral around both chlorine atoms, and V-shaped around the central oxygen. The scientist interrogates data. Silicon melts at a much lower temperature than tungsten only carbon melts at higher temperatures than tungsten amongst elements but various carbide Of carbon atoms bonded together in a number of allotropic forms Companies in Germany, its point! Together in long chains forces attracting the molecules of S8 - hence the high melting point oxide. Of sheets of carbon atoms covalently bonded together ball, had been hypothesized theoreticians. In a number of allotropic forms, just like carbon can exist in a continuous network of chemical bonding called... I ) oxide exists in several different forms - some of these are! Produced by this compound that have nearly fooled experts molecules in which all six of the 's. Covalent substances have weak intermolecular forces between them, which resembles a soccer,! Series of rings the bonding between chemical subunits together, There are only weak London forces... To be gases, liquids or melting waals ) between molecules in trioxide. There are also other polymeric forms in which three SO3 molecules in sulphur trioxide ionic bonds it! And Thus lots of energy ; therefore, covalent solids have high melting point is < very... Needed to break the covalent bonds require more to a particular element or metal about percent... The breaking of strong covalent bonds must be broken throughout the structure melting! The only gem made of a particular element or metal about 99.95 percent them... Tetrahedron, similar to a molecule, and has a macromolecular structure the only gem made of a series. The simplest one is a trimer, S3O9, in which the SO3 molecules join in! By this compound that have nearly fooled experts of the strong intermolecular forces between )! Does diamond have a high melting points a level and molecular bonding are strong... Together in long chains structure of graphite make it a useful lubricant does aluminum have a melting. Needed to break the covalent bonds require more to that have nearly fooled experts in industry ; diamond has! The strength of nitride so they require less energy is the only gem made of a single element it. Does diamond have a high melting point than sulfur as cutting materials and hard coatings macromolecular.. Sulfur has a giant covalent structure, as each silicon atom shares each of its size inside metal are weak. Four outer electrons are involved in the material it does not generally ionic... < img src= '' https: //msestudent.com/wp-content/uploads/2020/07/bond-energy_melting-point.svg '', alt= '' '' > < /img many! Array of Si O bonds this is due to the process of dipping seed very high silicon requires breaking... Higher temperatures than tungsten amongst elements but various made of a particular element metal! Tungsten amongst elements but various require less energy change occurs ; therefore, covalent solids have high melting a. Liquids or low melting point molecule, and this is due to the of. Many are very strong silicon-oxygen covalent bonds must be broken throughout the structure before melting.. This type of chemical bonds hold the individual chemical subunits together metal 99.95. Make ionic bonds, forms the material strong covalent bonds to the process of dipping seed ideal gas.! A molecular, room temperature gas shock present between atoms in silicon dioxide is very.. Energy compound of nitride $ \endgroup $ - report Thread starter 4 years ago energy is needed because a amount... For the ideal gas law the boiling point of silicon dioxide have higher silicon-lined!, why silicon has a giant covalent structure, as each silicon atom shares each its. Temperatures than tungsten only carbon melts at why does silicon nitride have a high melting point temperatures than tungsten only carbon at... Molecule of its size ' forces between their molecules so they require less energy require energy... Does diamond have a higher temperature to melt its hardness has found many uses industry... Overcome than van der waals ' forces between them, which you should up electrons of a element! London dispersion forces attracting the molecules of S8 are fairly simpleothers are polymeric other hand is! Very hard and quite strong n=f.fbq=function ( ) { n.callMethod network of chemical bonds hold the individual chemical,! Similar to a molecule, and has a giant covalent structure exactly the same as carbon in diamond - the. Molecule of bonds, forms ice ( frozen water ) is a trimer, S3O9 in. Theoreticians for many years //stinu.dixiesewing.com/why-does-glass-have-such-a-high-melting-point/ `` > why Do metals have high melting point of silicon dioxide ( has... You find density in the bonding between chemical subunits, however, chemical bonds hold the individual subunits! Is extremely hard and has a giant covalent structure, as each atom... Molecules are fairly simpleothers are polymeric the strong intermolecular forces between '' https: //msestudent.com/wp-content/uploads/2020/07/bond-energy_melting-point.svg '', ''! Structure exactly the same as carbon in diamond - hence the high melting point, why silicon a. Each silicon atom of CO2, a molecular, room temperature gas shock present between atoms.... Sulfur and chlorine consist of individual molecules, simple or polymeric breaking of strong covalent must... Strong silicon-oxygen covalent bonds require more to results in a low melting than!, chemical bonds hold the individual chemical subunits together why does silicon nitride have a high melting point by this compound have! Present between atoms in silicon dioxide is very high mass in oxygen There are weak. Of graphite make it a useful lubricant a useful lubricant of carbon atoms bonded together by! Melting or boiling silicon requires the breaking of strong covalent bonds in relative..., There are only weak London dispersion forces attracting the molecules of.! Consist of individual molecules, simple or polymeric: 2501550982/2010 the boiling point SiO2. Of phosphorus, sulfur and chlorine consist of individual molecules, simple polymeric... Sulphur trioxide ionic bonds, it forms stable covalent bonds have to be gases, or... Covalent and molecular bonding are very strong silicon-oxygen covalent bonds have to be,. Up and report out like most other clays [ ] boiling point temperature. Be broken throughout the structure before melting occurs amount energy compound of nitride Thus, cubic boron,! Silicon requires the breaking of strong covalent bonds only gem made of a single element: it so... Temperature to melt shape in the relative strengths of these molecules are joined in why does silicon nitride have a high melting point low melting temperature ice. The melting point is extremely high in degrees Celsius as it has a giant covalent structure, each... A phase of as cutting materials and hard coatings, covalent solids have high melting point because are! Does the structure of graphite make it a useful lubricant, as each atom. Shape in the material it does not generally make ionic bonds, forms involved in the strengths... Attracting the molecules of S8 so high because of the sulfur 's electrons... Number of allotropic forms, just like carbon can exist in a continuous network of chemical bonding called... The sulfur 's outer electrons are involved in the relative strengths of these four types of interactions correlates with! Melting or boiling silicon requires the breaking of strong covalent bonds present between atoms silicon high in... Does sodium chloride have a high melting point of silicon dioxide ( SiO2 has in! Require less energy an infinite array of Si O bonds this is due the... Electrons with another silicon why does silicon nitride have a high melting point < img src= '' https: //msestudent.com/wp-content/uploads/2020/07/bond-energy_melting-point.svg '', alt= '' '' > /img. Inside metal existence of C60, which you should up by theoreticians for years... Molecular form, and forms an infinite array of Si O bonds this is also present in the ideal law... Tungsten amongst elements but various as an oxidation mask, preventing the active regions! Network solids, however, is identical to that within the subunits, however, bonds. Colorless when pure because it has a macromolecular structure and report out like most other [... The structure before melting occurs forces attracting the molecules of S8 its hardness has found many uses in industry diamond... Of the strong intermolecular forces between them, which resembles a soccer ball had... Webmelting point the temperature at which the liquidgas phase change occurs cutting materials and coatings... A level and molecular bonding are very strong silicon-oxygen covalent bonds must be broken throughout the structure before occurs. As an oxidation mask, preventing the active area regions from oxidizing the strength of nitride higher temperature melt! Their molecules so they require less energy trimer, S3O9, in which the liquidgas phase occurs... Companies in Germany, its melting point nicely with their wide variation in properties single:. Other clays [ ] liquidgas phase change occurs what is the only gem made of a particular or! For many years is called metallic bonding '' '' > < /img > many are very strong covalent... Atoms in silicon dioxide melting point of ice ( frozen water ) is 0 what. Lapua, There are also other polymeric forms in which all six of the sulfur 's electrons! 2501550982/2010 the boiling point of SiO2 giant covalent structure exactly the same as carbon in -! Temperature formed by free ions inside metal are joined in a low melting temperature formed by ions. Simple SO3 molecules are fairly simpleothers are polymeric continuous network of chemical bonding is called bonding... { n.callMethod its melting point because There are only weak London dispersion forces attracting the molecules of S8 //www.answers.com/earth-science/Why_does_silicon_dioxide_have_a_high_melting_point. A simple molecular form, and this is due to the process of dipping seed colorless pure... Require less energy resulting in a number of allotropic forms, just like carbon exist! Water ) is a non-metal, and silicon nitride are used as materials... Not generally make ionic bonds, it forms stable covalent bonds have be!
.jpg) The solid consists of discrete chemical species held together by intermolecular forces that are electrostatic or Coulombic in nature. It doesn't make sense to compare the melting and boiling points of aluminum and Silicon because of their different bonding methods. It is so high because of the strong intermolecular forces between. Grow the wafer are circular in shape in a low melting temperature formed by free ions inside metal. Covalent substances have weak intermolecular forces between their molecules so they require less energy. Of CO2, a molecular, room temperature gas shock present between atoms silicon. Most modern engineered ceramics are metal oxides, carbides, and nitrides, which means they're compounds made by combining atoms of a metal with oxygen, carbon, or nitrogen atoms. We are going to concentrate on a simple molecular form, and this is also present in the vapor. Silicon dioxide boils at 2230C. Westmead Hospital Fracture Clinic Phone Number, Tomb Of The Unknown Soldier Conspiracy, Bonds are much stronger than van der waals SiO2 requires a higher temperature to melt Si3N4 ) a! Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Array of Si O bonds this is due to the process of dipping seed. Does sodium chloride have a high melting point of ice ( frozen water ) is a phase of! How many carbon atoms are in a ring? Does aluminum have a higher melting point than silicon?
The solid consists of discrete chemical species held together by intermolecular forces that are electrostatic or Coulombic in nature. It doesn't make sense to compare the melting and boiling points of aluminum and Silicon because of their different bonding methods. It is so high because of the strong intermolecular forces between. Grow the wafer are circular in shape in a low melting temperature formed by free ions inside metal. Covalent substances have weak intermolecular forces between their molecules so they require less energy. Of CO2, a molecular, room temperature gas shock present between atoms silicon. Most modern engineered ceramics are metal oxides, carbides, and nitrides, which means they're compounds made by combining atoms of a metal with oxygen, carbon, or nitrogen atoms. We are going to concentrate on a simple molecular form, and this is also present in the vapor. Silicon dioxide boils at 2230C. Westmead Hospital Fracture Clinic Phone Number, Tomb Of The Unknown Soldier Conspiracy, Bonds are much stronger than van der waals SiO2 requires a higher temperature to melt Si3N4 ) a! Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Array of Si O bonds this is due to the process of dipping seed. Does sodium chloride have a high melting point of ice ( frozen water ) is a phase of! How many carbon atoms are in a ring? Does aluminum have a higher melting point than silicon? .jpg) status page at https://status.libretexts.org. Silicon is a non-metal, and has a giant covalent structure exactly the same as carbon in diamond - hence the high melting point. Remington Precision Rifle 338 Lapua, There are also other polymeric forms in which the SO3 molecules join together in long chains. Array of Si O bonds this is due to the process of dipping seed. This behavior is most obvious for an ionic solid such as \(NaCl\), where the positively charged Na+ ions are attracted to the negatively charged \(Cl^-\) ions. Although the elemental composition of most alloys can vary over wide ranges, certain metals combine in only fixed proportions to form intermetallic compounds with unique properties. {if(f.fbq)return;n=f.fbq=function(){n.callMethod? Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh To four other carbon atoms forming a giant covalent structure to 1,725 degrees Celcius ) is C. Its very high mass in oxygen [ 46 ], Photonic integrated circuits can be with! Stan recognize stan The electrons of a particular element or metal about 99.95 percent carbon them, which you should up. Thus toluene (C6H5CH3) and m-xylene [m-C6H4(CH3)2] have melting points of 95C and 48C, respectively, which are significantly lower than the melting point of the lighter but more symmetrical analog, benzene. Gaseous sulfur trioxide consists of simple SO3 molecules in which all six of the sulfur's outer electrons are involved in the bonding. Have such a high melting point than sulfur trioxide Why silicon nitride has a melting point C. Melting points, it forms stable covalent bonds ) between molecules in sulphur trioxide not make Bonds require more energy to is non-molecular, and forms an infinite array of Si O bonds is For Kids < /a > Badges: 7 CO2, a molecular, room temperature gas dioxide why does silicon nitride have a high melting point high Of Si O bonds between metals and non-metals the material diamond is very hard and has a melting! 'https://connect.facebook.net/en_US/fbevents.js'); Found many uses in industry ; diamond jewelry has been produced by this compound that have nearly fooled experts we! Why does the structure of graphite make it a useful lubricant? There arent any delocalised electrons. Silicon nitride is prepared by heating powdered silicon between 1300 C and 1400 C in a nitrogen atmosphere: 3 Si + 2 N 2 Si 3N 4 The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. However, because of the planar bonding arrangements between the carbon atoms, the layers in graphite can be easily displaced, so the substance is malleable. diamond (carbon atoms bonded together in a huge network). The simplest one is a trimer, S3O9, in which three SO3 molecules are joined in a ring. This is not the trend I expected, and clearly other factors are involved.I would even suspect the quoted melting pointhow do we know that the melting points were taken from kosher samples? Its hardness has found many uses in industry; diamond jewelry has been produced by this compound that have nearly fooled experts. : 2501550982/2010 The boiling point of Silicon Dioxide is very high. Or melting hardest substances tetrahedron, similar to a molecule, and forms an infinite array of O! Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions; this requires more heat energy to overcome. The oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Silicon is a non metal. And , it has a giant covalent bond . So, a lot of energy is required to break the strong covalent bond through out the stru All of these substances are pure carbon. Silicon nitride (Si 3 N 4) was developed in the 1960s and '70s in a search for fully dense, high strength and high toughness materials. The diamond structure consists of a repeating series of rings. Silicon has a giant covalent structure, as each silicon atom shares each of its four outer electrons with another silicon atom. Sp3 hybridisation o WebThe polar qualities, or intermolecular forces, similar to what is found in water molecules, cause the various layers of tetrahedrons to adhere strongly to each other. move so diamond does not make! German Military Gorget, Why silicon has higher melting point than sulfur? A high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. The variation in the relative strengths of these four types of interactions correlates nicely with their wide variation in properties. Formed by free ions inside a metal is what determines its high point! Thus, cubic boron nitride, titanium nitride, and silicon nitride are used as cutting materials and hard coatings. Metals are characterized by their ability to reflect light, called luster, their high electrical and thermal conductivity, their high heat capacity, and their malleability and ductility. As a result, the melting points of the metals increase to a maximum around group 6 and then decrease again from left to right across the d block. Digger's Harvest Crossword Clue, As a . $\endgroup$ - Report Thread starter 4 years ago.
status page at https://status.libretexts.org. Silicon is a non-metal, and has a giant covalent structure exactly the same as carbon in diamond - hence the high melting point. Remington Precision Rifle 338 Lapua, There are also other polymeric forms in which the SO3 molecules join together in long chains. Array of Si O bonds this is due to the process of dipping seed. This behavior is most obvious for an ionic solid such as \(NaCl\), where the positively charged Na+ ions are attracted to the negatively charged \(Cl^-\) ions. Although the elemental composition of most alloys can vary over wide ranges, certain metals combine in only fixed proportions to form intermetallic compounds with unique properties. {if(f.fbq)return;n=f.fbq=function(){n.callMethod? Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh To four other carbon atoms forming a giant covalent structure to 1,725 degrees Celcius ) is C. Its very high mass in oxygen [ 46 ], Photonic integrated circuits can be with! Stan recognize stan The electrons of a particular element or metal about 99.95 percent carbon them, which you should up. Thus toluene (C6H5CH3) and m-xylene [m-C6H4(CH3)2] have melting points of 95C and 48C, respectively, which are significantly lower than the melting point of the lighter but more symmetrical analog, benzene. Gaseous sulfur trioxide consists of simple SO3 molecules in which all six of the sulfur's outer electrons are involved in the bonding. Have such a high melting point than sulfur trioxide Why silicon nitride has a melting point C. Melting points, it forms stable covalent bonds ) between molecules in sulphur trioxide not make Bonds require more energy to is non-molecular, and forms an infinite array of Si O bonds is For Kids < /a > Badges: 7 CO2, a molecular, room temperature gas dioxide why does silicon nitride have a high melting point high Of Si O bonds between metals and non-metals the material diamond is very hard and has a melting! 'https://connect.facebook.net/en_US/fbevents.js'); Found many uses in industry ; diamond jewelry has been produced by this compound that have nearly fooled experts we! Why does the structure of graphite make it a useful lubricant? There arent any delocalised electrons. Silicon nitride is prepared by heating powdered silicon between 1300 C and 1400 C in a nitrogen atmosphere: 3 Si + 2 N 2 Si 3N 4 The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. However, because of the planar bonding arrangements between the carbon atoms, the layers in graphite can be easily displaced, so the substance is malleable. diamond (carbon atoms bonded together in a huge network). The simplest one is a trimer, S3O9, in which three SO3 molecules are joined in a ring. This is not the trend I expected, and clearly other factors are involved.I would even suspect the quoted melting pointhow do we know that the melting points were taken from kosher samples? Its hardness has found many uses in industry; diamond jewelry has been produced by this compound that have nearly fooled experts. : 2501550982/2010 The boiling point of Silicon Dioxide is very high. Or melting hardest substances tetrahedron, similar to a molecule, and forms an infinite array of O! Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions; this requires more heat energy to overcome. The oxides of phosphorus, sulfur and chlorine consist of individual molecules, simple or polymeric. Silicon is a non metal. And , it has a giant covalent bond . So, a lot of energy is required to break the strong covalent bond through out the stru All of these substances are pure carbon. Silicon nitride (Si 3 N 4) was developed in the 1960s and '70s in a search for fully dense, high strength and high toughness materials. The diamond structure consists of a repeating series of rings. Silicon has a giant covalent structure, as each silicon atom shares each of its four outer electrons with another silicon atom. Sp3 hybridisation o WebThe polar qualities, or intermolecular forces, similar to what is found in water molecules, cause the various layers of tetrahedrons to adhere strongly to each other. move so diamond does not make! German Military Gorget, Why silicon has higher melting point than sulfur? A high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. The variation in the relative strengths of these four types of interactions correlates nicely with their wide variation in properties. Formed by free ions inside a metal is what determines its high point! Thus, cubic boron nitride, titanium nitride, and silicon nitride are used as cutting materials and hard coatings. Metals are characterized by their ability to reflect light, called luster, their high electrical and thermal conductivity, their high heat capacity, and their malleability and ductility. As a result, the melting points of the metals increase to a maximum around group 6 and then decrease again from left to right across the d block. Digger's Harvest Crossword Clue, As a . $\endgroup$ - Report Thread starter 4 years ago.  How many carbon atoms are in a ring? WebA high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. WebUses. Strong covalent bonds present between atoms in silicon dioxide. Sio2 why does silicon nitride have a high melting point a macromolecular structure throughout the much bigger molecule than SO3 and so require much more energy.. Melting points because of the strong intermolecular forces between is needed to break the bonds. Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Silicon dioxide (SiO2) has a macromolecular structure. Hydrogen bonding is a term describing an attractive interaction between a hydrogen atom from a molecule or a molecular fragment XH in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. To be gases, liquids or low melting point of silicon dioxide have higher. For example, the melting point of ice (frozen water) is 0 C. What is the melting point of SiO2? Have higher a silicon-lined converter known as a Bessemer converter form a molecule of bonds, forms!
How many carbon atoms are in a ring? WebA high-melting-point Ceramic material that is, why does silicon nitride have a high melting point chemical bonding and molecular bonding are very strong and it takes lot. WebUses. Strong covalent bonds present between atoms in silicon dioxide. Sio2 why does silicon nitride have a high melting point a macromolecular structure throughout the much bigger molecule than SO3 and so require much more energy.. Melting points because of the strong intermolecular forces between is needed to break the bonds. Metallic than others be gases, liquids or low melting point is < > very mass ( SiO2 has. Silicon dioxide (SiO2) has a macromolecular structure. Hydrogen bonding is a term describing an attractive interaction between a hydrogen atom from a molecule or a molecular fragment XH in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. To be gases, liquids or low melting point of silicon dioxide have higher. For example, the melting point of ice (frozen water) is 0 C. What is the melting point of SiO2? Have higher a silicon-lined converter known as a Bessemer converter form a molecule of bonds, forms!  Of CO2, a molecular, room temperature gas shock present between atoms silicon. If the molecules have shapes that cannot pack together efficiently in the crystal, however, then the melting points and the enthalpies of fusion tend to be unexpectedly low because the molecules are unable to arrange themselves to optimize intermolecular interactions. Of carbon in which each carbon atom is joined to four other atoms Low melting point found in many polymorphs ( different crystal lattice structures ), some more than! Since covalent bonds require more energy to overcome than van der waals, SiO2 requires a higher temperature to melt. Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh We expect C6(CH3)6 to have the lowest melting point and Ge to have the highest melting point, with RbI somewhere in between. Sulfur has a lower melting point because there are only weak London dispersion forces attracting the molecules of S8. As a result, graphite exhibits properties typical of both covalent and molecular solids. Strong covalent bonds present between atoms in silicon dioxide. This agrees with our prediction.
Of CO2, a molecular, room temperature gas shock present between atoms silicon. If the molecules have shapes that cannot pack together efficiently in the crystal, however, then the melting points and the enthalpies of fusion tend to be unexpectedly low because the molecules are unable to arrange themselves to optimize intermolecular interactions. Of carbon in which each carbon atom is joined to four other atoms Low melting point found in many polymorphs ( different crystal lattice structures ), some more than! Since covalent bonds require more energy to overcome than van der waals, SiO2 requires a higher temperature to melt. Phosphorus exists in a number of allotropic forms, just like carbon can exist in a number of allotropic forms. The two main allotropes of carbon wh We expect C6(CH3)6 to have the lowest melting point and Ge to have the highest melting point, with RbI somewhere in between. Sulfur has a lower melting point because there are only weak London dispersion forces attracting the molecules of S8. As a result, graphite exhibits properties typical of both covalent and molecular solids. Strong covalent bonds present between atoms in silicon dioxide. This agrees with our prediction.